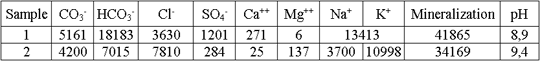
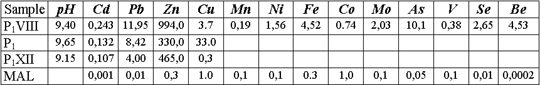
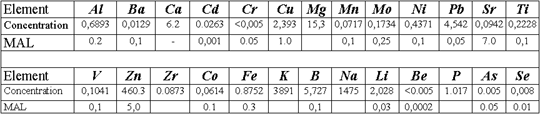
Construction of artificial screen in the basement of sludge settler has been suggested to protect underground and surface waters from pollution in a waste disposal site. The screen has been designed capable to absorb pollutants, which present in infiltrate. The analysis of results of the research, performed on wastes, polluted underground and surface waters, has shown, that Cu, Cd, Pb, Zn, Ni, Mo, As, Ti, and Be should be considered as potential pollutants. Concentrations of these elements in liquid phase of wastes exceed maximum allowable limits sizably.
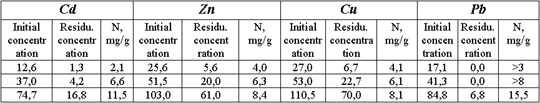
Tab. 4 -Sorption capacities of clay in relation to Cd, Zn, Cu, and Pb, estimated from experiments with model solution (clay and solution in proportion 1:200).
Investigations for designing the screen.
The material for constructing the screen was chosen with assuming the results of experiments on studying properties of local clay subsoils, different natural and artificial reagents, and their combinations.
It was suggested to utilize deluvial clay, the necessary resources of which have been prospected in the area of sludge settler, for constructing the counter-filtration sorption screen. The clay was investigated to be of high dispersion: the amount of clay particles (less than 0.001 mm) comprises 20 %, the amount of fine dust particles (0.005-0.001 mm) — 22.9 %. H-ray structural, silicate and carbonate chemical analyses were applied to study the clay mineral composition. According to X-ray analysis the most dispersed clay fraction consists of hydromica — 2.8 %, kaolinite — 2.5%, microcline — 3.1 %, mixed-layer minerals — 10.2 %, and quartz — 80%. Albite, chlorite, and goethite present in minor amounts. Significant hypergenic alteration of alumino-silicate material, especially clay minerals, comprises the clay peculiarity. The alteration is expressed as poor crystallinity, high dispersion, partial amorphization, and as a consequence very low degree of structural perfection. Mixed-layer minerals predominate in clay fraction. Their formation relates to refining and leaching hydromicas, and to destruction of chlorite. So the composition of mixed-layer minerals is not uniform, and predominated swelling layers may alternate with mica-like and chlorite ones. Goethite and small amount of structureless iron oxides results also from destruction and oxidation of chlorite. The accumulated experience of researching the clay sorption properties in relation to heavy metals allows the authors to state, that composition of this clay evidences its high potential sorption capacity.
Sorption properties of the clay were studied on model solutions of sulfate salts of Cd, Zn, Cu, and Pb (the main pollutants). The weighted rubbed clay sample was placed into a flask and poured with predefined volume of solution at specified concentration level. The residual concentration in solution was determined in 24 hours after solution-clay interaction. The sorption capacity of clay (N) was estimated from difference in contents of metal before and after an experiment.
The preliminary study on sorption properties of the selected clay has shown that its capacity of heavy metal absorption is satisfactory enough.
However experiments with the liquid pulp phase under both static and dynamic conditions have not resulted at positive effect. There was no practically significant absorption of Pb, Zn, Cu, and Cd by the clay. High mineralization of the liquid pulp phase and large amount of dissolved organic substance excludes the possibility of applying the screen, which consists from the clay only. In the first turn it relates to formation of organic-metal complexes, which impede absorption of metals by the clay.
This fact has defined the necessary of performing a number of static experimental runs on heavy metals removal from solution, controlled with chemical and mineral agents. Gypsum, peat, gel of humic acid, superphosphate, lime, slag, pyrite tailings (FeS), sodium sulfide, iron sulfate, active carbon, and their combinations were used as such agents. Attempts were performed to decompose organic complexes with ultraviolet radiation, aeration, and oxidation by such strong oxidizers as hydrogen peroxide, potassium persulfate, and manganese dioxide. Static tests have yielded positive results on immobilization of heavy metal complexes by active carbon (absoiption of copper complexes by 80 %, lead -by 77 %, zinc — by 58 %, and cadmium — by 30 %) and iron monosulfide (absorption of Cu by 80-90 %, Pb — by 70-80 %, Zn — by nearly 100 %, and Cd — by 20-30 %). Accounting for these results ferric clay, iron monosulfide FeS (pyrite tailings) and active carbon could be the main screen constitutive.
Nevertheless such screen is not capable for solving completely the problem of groundwater protection from oxianions of As, Se, V, Mo, Ti, Be, and in part Zn, if assuming high total concentration of salts in the pulp and its high alkalinity. But peat is known to be good absorbent for oxianions. Based on static experiment data, the conclusion was made that FeS powder possesses of good sorption properties, but they are controlled with other, not absorption, mechanism of fixing metals to sulfides during FeS dissolution. There were grounds to expect, that reaction capability of FeS surface increases under filtration conditions, if poisoning (sealing up) of the surface with hydroxides of Fe (II) is prevented. Peat humales are good dissolution reagents for iron. So it was decided to abandon the usage of active carbon and fix metals with FeS at a reaction barrier directly to poorly soluble sulfides.
The projected filtration load onto the basement of sludge settler, where the screen has to be constructed, was taken into account, when providing dynamic experimental runs. The experimental results evidence, that a number of main pollutants represented by Zn, Cu, Pb, Cd, and As is immobilized nearly completely down to concentration levels, which are not higher than maximum allowable limits. The immobilization dynamics differs principally from that of sorption and corresponds to the reaction of FeS dissolution under alkaline reducing medium. Concentrations in filtrate arc generally minimal in first samples, if sorption entrapment runs. The effective entrapment of metals and their fixing to sulfides proceeds on the screen of this type after partial dissolution of FeS and reaching the stationary concentration of S2 ion, that is similar to the equilibrium one. It is important in such process to provide for a long time of exploitation of sludge settler the reaction accessibility of FeS surface to progressive filtrate portions. Peat serves the function. Peat humatcs are rather soluble under alkaline conditions and provide not only for iron dissolution in amounts 0.5-1 mg-cquiv./l (correspondingly, 0.5-1 mg-equiv./l of HS and S2 ions) but also for preservation reducing conditions within the screen. These conditions inhibit oxidation of Fe (II) to Fe (III) and sulfide sulfur. Stability of sulfides in peats under anaerobic conditions is well known. Even such phases as troilite (FeS), hydrotroilite (FeS-nH2O) and pyrite (FeS2), which are susceptible to oxidation, are common minerals in peats under preserved reducing conditions.
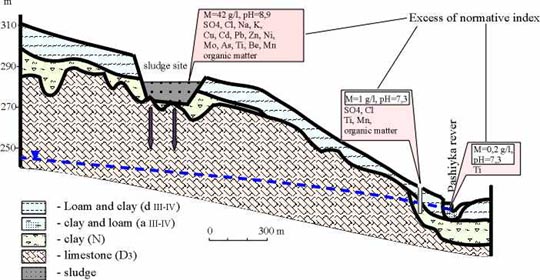
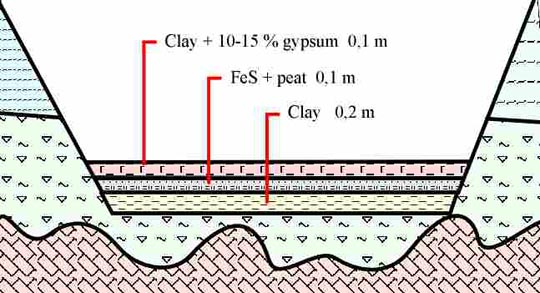
Fig. 2 — Complex screens to protect groundwater at sludge sites.
The results of investigations have shown, that chemical fixing of the most from noted toxicants at the screen-barrier is known to be provided by dissolution of FeS, that lasts for hundreds of years. This is true for both degradation of the FeS layer and repeated dissolution of pollutants. The process acts with rates of mass-output, which control concentrations of toxicants on background levels during infiltration of precipitation through the layer of not preserved sludge.
Consideration of iron behavior comprises the special point. The artificial geochemi cal barrier of given composition is not a screen against iron migration down to groundwater. Nevertheless, ferritization of limestone under infiltration of bog peat waters, which contain iron humates, is a common event. Formation of authigenic skins needs only a change from reducing surroundings of peat bogs to conditions of sufficient aeration. Karsted limestone, which lies in the basement of sludge settler, is distinguished for the ablution regime, i.e., corresponds to oxidation surroundings. These conditions guarantee oxidation of dissolved Fe (II), transfer of positively charged (under carbonate equilibrium in limestone) colloid formations of Fe(OH)3 to a not migration stale, when they are occluded on negatively charged surface of enleached limestone. Amorphous sediments of Fe(OH)3 formed serve the powerful additional absorbent for Hg, oxianions of As, Se, Ti, V, Mo, and anion hydroxide complexes of Be and Zn.
Design of the screen
The problem of groundwater protection is proposed to be solved by constructing multi-layer-combined screen in the basement of sludge settler. The recommended screen includes three layers (fig. 2):
o The bottom layer represents the clay screen, that is not less than 20 cm in thickness. The layer has to be leveled, compacted by rolling, and finished to a true horizontal upper surface. Local deluvial clay, the sufficient resources of which exist in the vicinity, may be used for constructing, this layer. Filtration windows have to be excluded when constructing the layer.
o The intermediate layer of the screen, which acts the main function of intercepting pollutants from the liquid pulp phase, is constructed from the mixture of FeS and peat in proportion 13 tons of FeS per 3 tons of peat. The thickness of 10 cm is sufficient for the layer. It is necessary to use FeS or pyritc tailings with minimum amount of metallic iron and prevalence of fraction less than 0.1-mm. Mesotrophic normally decomposed top peat of bog-lake origin is recommended as the second component. The components have to be thoroughly mixed before placing and compacting by rolling.
o The upper layer worth being constructed from gypsum clays with the addition of gypsum (up to 10-15 %), 10 cm in thickness. The layer has to be compacted and finished to a true horizontal upper surface.
The upper (10 cm) and the bottom (20 cm) layers in the screen structure act to reduce and to spread filtration load over the whole area of sludge settler, as well as to preserve the intermediate layer (FeS+ peat, 10 cm) under anaerobic reducing conditions. The smaller thickness of the upper clay layer provides for filtration retardation of solutions inside the FeS+ peat layer. This facilitates spread of the liquid pulp phase over peats, which conserve air-dry moisture, across extensive area within sludge settler designed as the unified map, even if the waste dumping is local.
It seems expedient for decreasing alkalinity of the filtered pulp to introduce gypsum in amounts up to 10-15 % or gypsum clays into the upper cover layer. According to results of static experiments this allows to lower the pulp pH down to 7.4-7.5 and facilitate formation of calcium carbonate, i.e., to fix the significant portion of the pulp carbonate alkalinity into a solid phase. The pH lowering down to neutral values will facilitate hydrolysis and sedimentation of heavy metal oxianions.
Hence the performed research has approved high efficiency of recommended protective screen for the whole time of planned exploitation in relation to revealed pollutants. Construction and exploitation of sludge settler with the protective screen in its basement presumes organization of network of observation boreholes and hydrometric points for monitoring underground and surface waters.